Business
Difference Between Ideal and Solution
Introduction
Solutions are a fundamental concept in chemistry and play a crucial role in various industries, from pharmaceuticals to petrochemicals. When it comes to solutions, they can be broadly categorized into two types: ideal and non-ideal solutions. These categories are essential for understanding how different substances interact in a mixture and how this affects their properties. In this article, we will explore the key differences between ideal and non-ideal solutions, shedding light on their behavior, properties, and real-world applications.
Ideal Solutions
Definition
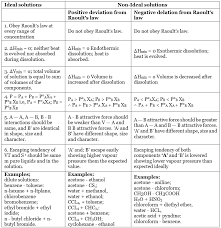
An ideal solution, also known as an ideal mixture, is a solution that exhibits ideal behavior when mixed. In an ideal solution, the intermolecular forces between the solvent and solute molecules are similar in strength and nature. This means that the interactions between the molecules in the solution are uniform and consistent.
Characteristics
- No Energy Change: In an ideal solution, there is no energy change when the solute is dissolved in the solvent. The enthalpy change is zero, signifying that there is no heat evolved or absorbed during the mixing process.
- Raoult’s Law: Ideal solutions obey Raoult’s Law, which states that the vapor pressure of each component in the solution is directly proportional to its mole fraction. This means that the vapor pressure of each component is directly predictable from its concentration.
- Ideal Mixing: Ideal solutions mix seamlessly and without volume or enthalpy changes. This is because the intermolecular forces between the solute and solvent are similar.
- No Deviation from Beer’s Law: Ideal solutions also do not deviate from Beer’s Law, which relates the absorbance of a solution to its concentration.
Real-World Example
One common example of an ideal solution is the solution of ethanol and water. These two substances have similar intermolecular forces, and when mixed, they form an ideal solution. The mixing of ethanol and water results in a solution with properties that are predictable based on their concentrations.
Non-Ideal Solutions
Definition
In contrast to ideal solutions, non-ideal solutions are those in which the interactions between the solute and solvent molecules are not uniform. The intermolecular forces between the components in a non-ideal solution are different in strength and nature, leading to deviations from ideal behavior.
Characteristics
- Enthalpy Deviation: Non-ideal solutions exhibit enthalpy changes upon mixing. This means that heat is either evolved or absorbed during the dissolution process. The enthalpy change can be positive or negative, depending on the specific interactions between the components.
- Non-Ideal Mixing: Non-ideal solutions do not mix perfectly, leading to changes in volume and enthalpy. These changes are often due to variations in intermolecular forces between the solute and solvent.
- Deviations from Raoult’s Law: Non-ideal solutions deviate from Raoult’s Law. This means that the vapor pressure of the components is not directly proportional to their mole fractions. The observed vapor pressure differs from the predicted values based on Raoult’s Law.
- Deviations from Beer’s Law: Non-ideal solutions can also deviate from Beer’s Law. The relationship between concentration and absorbance is not linear, making it challenging to accurately determine the concentration of the solute.
Real-World Example
A classic example of a non-ideal solution is the mixing of acetone and chloroform. These two substances have dissimilar intermolecular forces, leading to non-ideal behavior when combined. The enthalpy change during the mixing process is not zero, and deviations from Raoult’s Law and Beer’s Law can be observed.
Key Differences Between Ideal and Non-Ideal Solutions
Now that we have discussed the characteristics of ideal and non-ideal solutions, let’s highlight the key differences between the two:
- Intermolecular Forces: In ideal solutions, the solute and solvent molecules have similar intermolecular forces, while in non-ideal solutions, these forces are different.
- Enthalpy Change: Ideal solutions have zero enthalpy change upon mixing, while non-ideal solutions exhibit enthalpy changes.
- Raoult’s Law: Ideal solutions follow Raoult’s Law, while non-ideal solutions deviate from it.
- Mixing Behavior: Ideal solutions mix without volume or enthalpy changes, whereas non-ideal solutions exhibit changes in volume and enthalpy.
- Predictability: Ideal solutions are highly predictable in terms of properties based on concentrations, while non-ideal solutions are less predictable due to deviations from ideal behavior.
Real-World Applications
The distinction between ideal and non-ideal solutions has significant implications in various fields:
- Chemical Engineering: Understanding ideal and non-ideal behavior is crucial in designing and optimizing chemical processes.
- Pharmaceuticals: Drug formulations often involve the dissolution of active pharmaceutical ingredients in solvents, making knowledge of solution behavior vital.
- Environmental Science: Knowledge of non-ideal solutions is essential in environmental science, especially when studying the behavior of pollutants in natural waters.
- Food Science: The behavior of solutes in food products, such as sugars in beverages, can be understood through the concept of ideal and non-ideal solutions.
Conclusion
In conclusion, the difference between ideal and non-ideal solutions lies in the uniformity of intermolecular forces and the predictability of properties. Ideal solutions are characterized by similar intermolecular forces, while non-ideal solutions exhibit variations in these forces, leading to deviations from Raoult’s Law and Beer’s Law. Understanding these differences is crucial in various scientific and industrial applications, from chemical engineering to pharmaceuticals. It allows scientists and engineers to make informed decisions about the behavior of solutions, ultimately leading to more effective processes and product development.
FAQs
- Can a solution be both ideal and non-ideal at the same time? No, a solution is typically classified as either ideal or non-ideal based on the uniformity of intermolecular forces between its components.
- Are all real-world solutions either ideal or non-ideal? Yes, real-world solutions can be categorized as either ideal or non-ideal based on the nature of their intermolecular interactions.
- What are some other examples of ideal and non-ideal solutions in everyday life? Ideal solutions include ethanol-water mixtures, while non-ideal solutions include acetone-chloroform mixtures.
- How is the behavior of ideal and non-ideal solutions utilized in industry? Industries use this knowledge to optimize processes, such as chemical reactions and separation techniques, to achieve desired results efficiently.
- Are there any exceptions where a solution behaves as both ideal and non-ideal under different conditions? Some solutions may exhibit ideal behavior under certain conditions and non-ideal behavior under others, depending on factors like temperature and pressure.


