Tech
Zeotropic vs. Azeotropic Mixtures: the Differences
In the fascinating realm of thermodynamics and chemical engineering, the concepts of zeotropic and azeotropic mixtures hold a pivotal place. These terms describe the behavior of mixtures, particularly when it comes to phase transitions and distillation processes. In this article, we will delve into the differences between zeotropic and azeotropic mixtures, exploring their characteristics, properties, and real-world applications.
The Basics: Mixtures and Distillation
Before we dive into zeotropic and azeotropic mixtures, let’s start with some fundamentals. A mixture is a combination of two or more substances that are physically intermingled but not chemically combined. In the context of distillation, mixtures are often separated into their individual components based on differences in boiling points.
Distillation is a separation process that involves heating a liquid mixture to create vapor and then cooling the vapor to create a liquid. This process exploits the fact that components in a mixture have different boiling points, allowing for their separation.
Zeotropic Mixtures
A zeotropic mixture is a mixture of substances that do not boil at a constant temperature. In other words, as the mixture is heated, the composition of the vapor phase is different from the composition of the liquid phase. This non-constant boiling behavior is a result of the varying vapor pressures of the components in the mixture.
Characteristics of Zeotropic Mixtures:
- Changing Composition: In a zeotropic mixture, the composition of the vapor phase changes as the mixture is heated. This makes it particularly challenging to separate the components effectively through distillation.
- Fractional Distillation: Zeotropic mixtures often require a process known as fractional distillation, which involves multiple distillation steps to achieve separation.
- Examples: Common refrigerants like R-410A and R-404A are zeotropic mixtures used in air conditioning systems.
- Key Differences
- Let’s highlight the key differences between zeotropic and azeotropic mixtures:
- Boiling Behavior: Zeotropic mixtures have varying boiling points, while azeotropic mixtures have a constant boiling point.
- Composition Change: Zeotropic mixtures exhibit a changing composition between the vapor and liquid phases during heating, whereas azeotropic mixtures maintain a consistent composition.
- Separation Difficulty: Zeotropic mixtures are more amenable to separation using distillation techniques, while azeotropic mixtures are challenging to separate via conventional distillation.
- Real-World Applications
- Understanding zeotropic and azeotropic mixtures is crucial in various industrial applications:
- Chemical Industry: Distillation processes are common in the chemical industry for the separation of different components in mixtures.
- Petroleum Refining: The separation of hydrocarbons in crude oil relies on distillation processes, and knowledge of zeotropic and azeotropic behaviors is essential.
- Pharmaceuticals: Pharmaceutical companies use distillation to separate and purify various chemical compounds in drug manufacturing.
- Food and Beverage: The production of alcoholic beverages involves azeotropic mixtures like the ethanol-water mixture.
- Environmental Protection: Zeotropic and azeotropic refrigerants are used in air conditioning systems, with implications for environmental protection and regulation.
- Zeotropy in Refrigerants
- One notable application of zeotropy is in refrigerants used in air conditioning and refrigeration systems. Zeotropic refrigerants have become popular as alternatives to ozone-depleting substances like CFCs and HCFCs. They offer better environmental properties but also present challenges due to their changing compositions during the phase change process. Engineers and scientists work to optimize the design of refrigeration systems to accommodate zeotropic refrigerants effectively.
- Conclusion
- Zeotropic and azeotropic mixtures are fundamental concepts in the world of thermodynamics and chemical engineering, with significant implications for separation processes like distillation. Understanding their differences is crucial for various industries where the separation and purification of components in mixtures play a vital role.
- As technology and environmental considerations evolve, the development of innovative separation techniques and the use of zeotropic and azeotropic mixtures continue to shape the landscape of chemical processes and environmental sustainability.
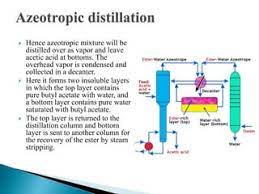
Azeotropic Mixtures
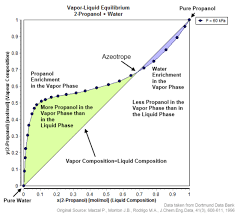
An azeotropic mixture is a mixture of substances that boils at a constant temperature, and the composition of the vapor phase is the same as the composition of the liquid phase. In simple terms, an azeotrope behaves as a single substance during distillation, making it challenging to separate its components by conventional distillation techniques.
Characteristics of Azeotropic Mixtures:
- Constant Boiling Point: Azeotropic mixtures have a fixed boiling point, and their vapor and liquid phases have the same composition at this temperature.
- Difficult Separation: Azeotropic mixtures are challenging to separate using traditional distillation methods because the vapor phase composition remains constant throughout the process.
- Examples: The ethanol-water azeotrope, with a composition of approximately 95.6% ethanol and 4.4% water, is used in the production of alcoholic beverages.
Key Differences
Let’s highlight the key differences between zeotropic and azeotropic mixtures:
- Boiling Behavior: Zeotropic mixtures have varying boiling points, while azeotropic mixtures have a constant boiling point.
- Composition Change: Zeotropic mixtures exhibit a changing composition between the vapor and liquid phases during heating, whereas azeotropic mixtures maintain a consistent composition.
- Separation Difficulty: Zeotropic mixtures are more amenable to separation using distillation techniques, while azeotropic mixtures are challenging to separate via conventional distillation.
Real-World Applications
Understanding zeotropic and azeotropic mixtures is crucial in various industrial applications:
- Chemical Industry: Distillation processes are common in the chemical industry for the separation of different components in mixtures.
- Petroleum Refining: The separation of hydrocarbons in crude oil relies on distillation processes, and knowledge of zeotropic and azeotropic behaviors is essential.
- Pharmaceuticals: Pharmaceutical companies use distillation to separate and purify various chemical compounds in drug manufacturing.
- Food and Beverage: The production of alcoholic beverages involves azeotropic mixtures like the ethanol-water mixture.
- Environmental Protection: Zeotropic and azeotropic refrigerants are used in air conditioning systems, with implications for environmental protection and regulation.
Zeotropy in Refrigerants
One notable application of zeotropy is in refrigerants used in air conditioning and refrigeration systems. Zeotropic refrigerants have become popular as alternatives to ozone-depleting substances like CFCs and HCFCs. They offer better environmental properties but also present challenges due to their changing compositions during the phase change process. Engineers and scientists work to optimize the design of refrigeration systems to accommodate zeotropic refrigerants effectively.
Conclusion
Zeotropic and azeotropic mixtures are fundamental concepts in the world of thermodynamics and chemical engineering, with significant implications for separation processes like distillation. Understanding their differences is crucial for various industries where the separation and purification of components in mixtures play a vital role.
As technology and environmental considerations evolve, the development of innovative separation techniques and the use of zeotropic and azeotropic mixtures continue to shape the landscape of chemical processes and environmental sustainability.